‘Ethical, impactful and better science’

6th December 2024
From grimace guides to ‘virtual dogs’, Dr Genevieve Barr looks at 20 years of innovations to help replace, reduce and refine the use of animals in research
The way scientists plan, perform and publish biomedical research has changed significantly over the past 20 years. Advances in technology have given researchers more options so they can choose the most relevant tool to tackle their scientific question, from personalised models of organs created from patients’ own cells to in silico approaches powered by AI and machine learning. As well as the power to provide unique insights into human biology and disease, these models have the potential to replace the use of animals in many fields of research.
Replacement is one pillar of the 3Rs, alongside reducing the number of animals involved in research and refining their care and use to maximise animal welfare and ensure that every animal used meaningfully contributes to the scientific knowledge base. At the NC3Rs we have been investing in new models and exploiting advances in technology and tools to replace, reduce and refine animal use since we were founded in 2004. We are the UK’s National Centre for the 3Rs and drive changes in policy, regulation and practice around the world.
As we celebrate two decades of innovation, we look back at how advances in the 3Rs have pioneered better science.
Simple changes
It is now widely accepted that mice experience a wide range of emotions including stress and anxiety, but 20 years ago some scientists did not think rodents could even feel pain. The scientific community’s understanding of refinement has developed over the past two decades and our refinement research encompasses the social drivers of stress¹, how animals communicate their emotional states² and the benefits of positive interactions with handlers, such as rat tickling³. We want to better understand how welfare affects animals’ emotional responses, behaviour and physiology, and as a result the reproducibility and relevance of research they are used in.
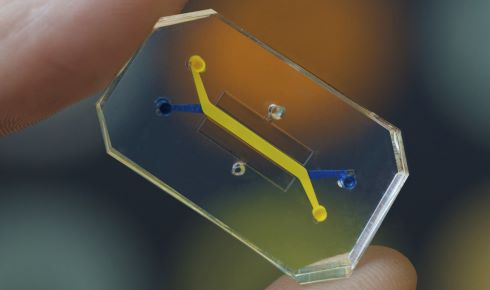 Organ on a chip technology.
Organ on a chip technology.
It is important to consider all aspects of how laboratory animals are housed, handled and cared for. A good example is refined mouse handling. Traditionally, mice have been picked up by the tail, but research funded by the NC3Rs has shown this causes stress and anxiety that can make scientific findings less reliable and reproducible⁴. The simple change of using a plastic tunnel or cupped hands makes a big difference to mouse welfare and scientific results. For example, tunnel-handled mice do better in behavioural tests compared with tail-handled mice, being more willing to explore mazes and investigate new objects⁵. In the 14 years since the initial research there is now overwhelming evidence for the benefits of cupping and tunnel handling, and our refined mouse handling resources⁶ have supported many institutions worldwide to change their practices to stop picking mice up by the tail, benefitting millions of mice used in scientific research and testing.
What seems simple can be difficult to put into practice and it can take a long time to build the evidence, confidence and skills needed across the life sciences for even small changes to become common practice. For example, giving animals pain relief seems straightforward, but can be difficult because prey animals such as mice, rats and rabbits that are used in research instinctively conceal signs of suffering to avoid predation in the wild.
To support better pain management for tens of millions of laboratory animals, we funded research showing that animals in pain show distinctive behaviours and facial expressions, which have been used to create ‘grimace scales’⁷. For example, almost all species (including humans) squint their eyes when they are in pain.
Some pain-related facial expressions can be subtle and hard to spot – for example, changes in whisker shape and position. We have produced easy-to-use grimace scale posters⁸ in six different languages so animal facilities around the world can use the simple scale to identify animals that need pain relief. We have also invested in an app that uses AI and machine learning to automatically detect when mice are showing signs of pain⁹, giving researchers and animal technicians new tools to take action as fast as possible.
The ripple effect
Over the past 20 years the perception of the 3Rs has shifted away from purely ethical principles to an integral framework for research that is robust, reproducible and meaningfully advances understanding of biology and disease – both for studies that use animals and those that replace them.
Everyone working in science today will be aware of the ‘reproducibility crisis’, a phrase coined in 2012 to describe the increasing recognition that many published results could not be reproduced by another, or even the same, scientist. Where animals are involved, studies that cannot be reproduced waste animal lives, as well as research funding, time and resources.
Transparent reporting and rigorous experimental design are crucial for reproducible in vivo research that uses the lowest number of animals to generate reliable results. We raised awareness of issues with the quality of animal research in 2009¹⁰, providing evidence of systematic flaws in the experimental design, statistical analysis and reporting of research using animals that are major contributors to a lack of reproducibility. Our Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines¹¹ published the following year are a set of recommendations to maximise the quality and reliability of published research involving animals, and enable others to better scrutinise, evaluate and reproduce it.
International perspective
A decade on from ARRIVE’s launch, we released ARRIVE 2.0 to support the improvements needed to make poor reporting of animal research a thing of the past. We have made the ARRIVE guidelines available in 10 additional languages¹² and are developing an AI tool to check manuscripts¹³ include the information needed to assess and reproduce the results.
Our Experimental Design Assistant (EDA)¹⁴ uses computer-based logical reasoning to provide bespoke feedback and advice, and plan robust animal experiments. ARRIVE and the EDA are important parts of the international momentum towards reproducible science and are endorsed by journals, funders and universities to improve the transparency and reproducibility of animal research.
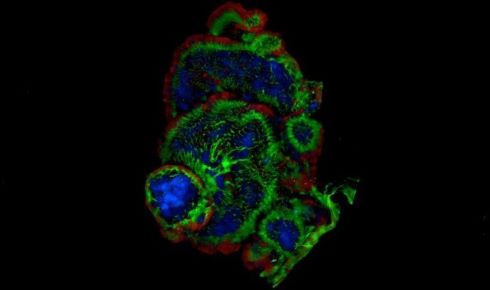 Organoids are helping scientists study development and tissue responses without direct experimentation on live animals.
Organoids are helping scientists study development and tissue responses without direct experimentation on live animals.
By taking an international perspective and acting as an ‘honest broker’ to connect scientists, industry, policymakers and regulators, we can achieve wholesale change in the way animals are used in scientific research and testing across sectors and around the world. For example, we led an international group of experts to review World Health Organization (WHO) guidance for the development, production and quality control of biologics such as vaccines, immunoglobulins, blood products and hormones.
Up to 10 million animals a year are used worldwide in these tests, which are expensive, can cause significant pain and distress to the animals, and can be highly variable, causing lengthy delays to the release of key medicines. In vitro alternatives are now available for some tests that reduce assay variability, time and resources required, and we have recommended new language for WHO guidelines to focus on replacement approaches where they are validated and available, and ensure refinements are included where they are not. Building on this, with further funding from the Bill & Melinda Gates Foundation, we are working to remove obsolete animal tests and develop replacement approaches where they are needed most urgently – for example, where animal tests cause significant suffering¹⁵. This is part of our work to build confidence in alternative approaches when the scientific and regulatory framework is traditionally built around animal use.
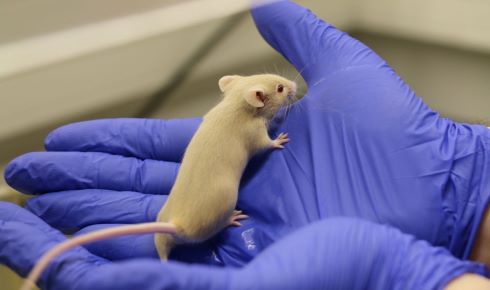 Picking mice up with cupped hands, rather than by the tail, makes a big difference to both their welfare and scientific results
Picking mice up with cupped hands, rather than by the tail, makes a big difference to both their welfare and scientific results
Challenging the status quo
Despite advances in science and technology giving scientists more options than ever to embed the 3Rs, there is still work to do to change perceptions and practice. Animal models are considered the de facto gold standard in many fields of research and their use is embedded and required by regulatory frameworks.
But many of the regulatory toxicology tests used today were introduced 30 or 40 years ago. The pharmaceutical industry has changed considerably since, with new drug targets, new types of compounds and new in vitro and in silico technologies available to evaluate safety. Currently, two animal species (a rodent and non-rodent) are typically used to test if new medicines are safe to give to humans in clinical trials.
Our Two Species project¹⁶ is reviewing this paradigm, in collaboration with over 30 pharmaceutical companies and regulatory bodies, to try to reduce the reliance on animals in drug testing. With support from the Association of the British Pharmaceutical Industry we first focused on biologics and showed that the second non-rodent species (typically dog, primate or minipig) could be avoided in longer-term toxicity tests. We are now working to build a robust case to support changes to regulatory guidelines and expand opportunities to use only a single species.
Dogs are the most commonly used non-rodent species for new small molecule drugs. Our Virtual Second Species project¹⁷ is developing a machine-learning-aided multi-scale modelling framework for safety-testing new medicines and chemicals, aiming to create a ‘virtual dog’ to replace their use for chronic toxicity studies.
The virtual dog is being developed as part of CRACK IT¹⁸, a unique funding competition launched in 2011 that connects scientists with pharmaceutical, chemical and consumer product companies to develop new 3Rs technologies that can have immediate impacts on animal use in the life science industry.
Like the Virtual Second Species, many challenges are ambitious, pushing the boundaries of what is possible and requiring cross-sector collaboration to solve. This includes developing in silico tools to predict hormone disruption and replace some animal toxicity tests, and developing real-time monitoring for organ-on-chips to track oxygen demand, protein secretion, cell signalling and enzymatic activity, improving the quality of data from these important replacement models.
The modern 3Rs
The ethos of the 3Rs is not settling for ‘the way things are done’. There is increasing recognition that replacement approaches can offer new scientific insights and model aspects of biology and disease that would not be possible with animal experiments. As well as ethical and scientific drivers, research that minimises or replaces animal use is often faster, cheaper and more sustainable.
As the understanding of animal emotion and experience expands, approaches to maximise animal welfare must adapt and the ethical framework around their use must evolve. It is impossible to predict what advances the next 20 years will bring or the new ethical dilemmas they will present. In the face of unknowns, the 3Rs remain the constant guiding principle for ethical, innovative, impactful – and better – science.
Dr Genevieve Barr is science manager (communications) at NC3Rs.
1) Do male mice prefer to live on their own? (NC3Rs)
3) Refinement of tickling protocols to improve positive animal welfare in laboratory rats (NC3Rs)
4) Taming anxiety and variation in laboratory mice (NC3Rs)
5) Gouveia, K. & Hurst, J. Optimising reliability of mouse performance in behavioural testing: the major role of non-aversive handling. Sci. Rep. 7, 44999 (2017)
6) Mouse handling (NC3Rs)
7) The assessment of pain using facial expressions in laboratory mice, rats, rabbits and macaques (NC3Rs)
8) Grimace scales (NC3Rs)
9) Mouse MApp (NC3Rs)
10) Kilkenny, C. et al. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS ONE 4(11): e7824 (2009).
12) Arrive guidelines translations
13) Arrive guidelines compliance checker
14) Helping researchers worldwide design robust and reliable experiments
15) Supporting 3Rs approaches in biologicals testing (NC3Rs)
16) Review of the use of two species in regulatory toxicology studies: Phase II – molecules following ICH M3(R2) (NC3Rs)
17) Virtual second species (NC3Rs)
18) Crack it challenges (NC3Rs)


