Lessons from larvae
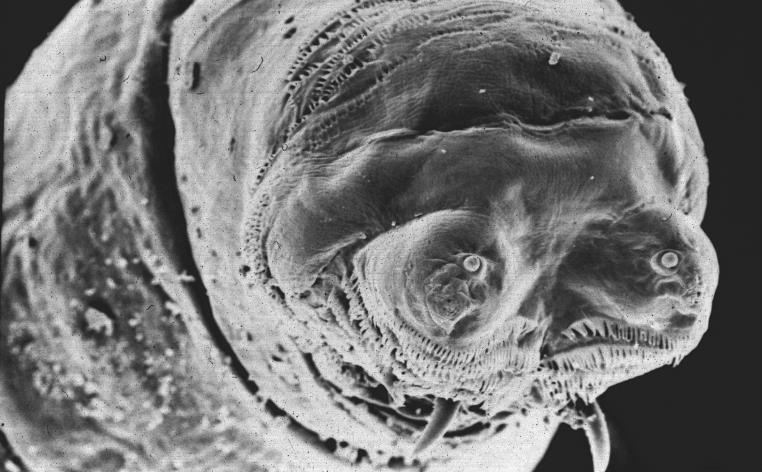
Biologist and author Professor Matthew Cobb explains why studying the much-maligned maggot can help us better understand ourselves – from our genes and development to our sense of smell
The Biologist 63(4) p12-15
Maggots have a bad reputation. Wriggling, amorphous things, they are generally seen as synonymous with decay. However, behind the horror-film images there lurks a biological marvel that has intrigued scientists for centuries and is now the source of fundamental discoveries with widespread implications.
The most widely studied maggot is the larva of the vinegar fly, Drosophila melanogaster. Drosophila have been the focus of genetic research for over a century: in the 1910s, they were used by Thomas Morgan at Columbia to show that genes are on chromosomes, and then by Morgan's student, Hermann Muller, who in 1926 showed that mutations could be induced by x-rays, thereby confirming the idea that genes had a material basis.
All of that work was done on flies, not maggots, but for many years, scientists were primarily interested in larvae because the cells of their salivary glands contain giant chromosomes that are easy to dissect and stain, revealing complex banding patterns that showed the location of genes in the decades before DNA sequencing.
In the 1970s, as molecular genetic techniques became increasingly widespread, Drosophila larvae were the focus of two areas of research in which they continue to be important: developmental biology and neuroscience. Scientists investigated how a fly egg turns into a maggot, revealing the patterning of developmental control genes ('Hox' genes) in the embryo, and the way that some parts of the developing embryo turned into patches of skin ('imaginal discs') within the maggot that would eventually become adult organs: eyes, legs, antennae and so on.
In 1995, Ed Lewis, Christiane Nüsslein-Volhard and Eric Wieschaus were awarded the Nobel Prize in Physiology or Medicine for their work on the genes that control embryonic development.
During the 1980s and 1990s, scientists developed models to explain how development occurs, using Alan Turing's ideas about morphogens – chemicals that diffuse through developing tissue – to explain how cells 'know' where they are in the tissue, and therefore what genes need to be activated to produce the relevant pattern. Many of these principles can be applied to development in other animals.
In neurobiology, the maggot was initially studied because it provided a simple way to investigate how nerves control muscles. Maggots are basically long tubes composed of repeated segments. In each segment, muscles in the body wall contract in forward-moving waves to produce the insect's wriggling movement. Those waves of activity are controlled by a series of nerves, which can be studied using electrophysiology. Neurotransmitters in the neuromuscular junction or synapse can be manipulated directly or by altering the genes that produce them or their receptors. As in so many aspects of Drosophila, the larval nerves can serve as a model for understanding the development and function of similar systems in other animals.
This link with humans is true not only for how these synapses function, but also how they malfunction. The first human neurological disease to be described was epilepsy. A large number of Drosophila mutations induce seizures similar to those seen on a much larger scale in the human brain during an epileptic fit, and provide a way of understanding and perhaps treating the disease.
Many groups around the world, including my colleague at Manchester, Richard Baines, are using maggots and the power of Drosophila genetics – which enables us to turn genes on and off at will and to express genes from other species in precisely identified tissues – to explore potential new drugs to treat epilepsy.
The work on the neuromuscular junction illustrates the advantages of the maggot. It is much simpler than the adult fly, making it amenable to studies of basic biological processes, but it retains all the advantages of Drosophila genetics. This principle – using a simple system to study something that is also present in more complex organisms – can also be seen in my work on the sense of smell in Drosophila maggots.
Although maggots and humans have very different olfactory receptors, the way the nose and the brain are wired up is essentially the same in all animals. Maggots show very straightforward behavioural responses to smells: they either move towards the odour source if they like it or away if they find the odour distasteful. Using simple behavioural measures (you put maggots on a jelly-covered plate together with an odour and watch them wriggle for five minutes), my group has identified genes involved in odour processing and developed neurobiological models of how the tiny maggot brain analyses and responds to smells.
By using Drosophila genetics, we can do something that is not possible in any other organism: we can make a maggot with just one functioning neuron in its 'nose'. You and I have about 4 million olfactory receptors, or smell cells, divided into about 400 types. A maggot has just 21 smell cells, each of which is different. By making a maggot with just one functioning smell cell, my colleague Catherine McCrohan and I, together with a number of students and researchers, have been able to study the electrical activity of these cells as they are stimulated.
This fastidious, delicate research – the downside of maggots is that they are very small, and their noses are far, far smaller – has enabled us to build up a picture of how smells are identified at the very earliest stage, even before the signal hits the brain.
Our research has revealed that, even in something as simple as a maggot, the smell cells are exquisitely sensitive. Each type of cell can respond to a range of odours, and each odour can be detected by a range of cells, producing what is called a combinatorial code. That code is nothing like a binary code – the nose cells are not a set of switches that are turned on or off. Instead, the same cell can be activated or inhibited by different odours, and can show differences both in the intensity of its response and in the duration and shape of that response.The signal in the nose is incredibly rich.
With our colleague Rasmus Petersen, we have used computer modelling to show that the activity of those smell cells contains not only intensity information, but also what is called temporal information – the nature of the odour is encoded by the overall shape and duration of the cell's response. Our next step will be to manipulate the activity of these cells using pieces of the Drosophila genetic toolkit to demonstrate that the brain uses this temporal information to guide the maggot's behaviour.
Although the simplicity of the maggot is so attractive, it turns out to be remarkably complicated. An international consortium of researchers around the world are currently drawing up the neuronal wiring diagram of the maggot. It has taken them years and they still have not finished.
In the early stages of their work, Albert Cardona discovered a mind-boggling degree of complexity that proves maggots are anything but tiny robots. For the maggot to move in a coordinated way, it has to detect that a movement has taken place. It does this through tiny stretch receptor cells that send a signal when the animal's body wall is extended. Each of these cells has 53 neurons coming into it, and it sends its signal out to another 18 cells and a further 74 cells called interneurons. Most if not all of these synapses contain more than one kind of neurotransmitter, enabling the cell to transmit information in complex and unknown ways – the computing power in each of these tiny cells is extraordinary.
This complexity – which can be found far from the maggot's intricate brain, and is involved in nothing more than controlling repetitive rhythmic movement – shows how even the simplest organism is far more complex than our most powerful computer. When we fully understand the structure and function of the maggot's brain and peripheral nervous system, we can expect to gain new insights into fundamental processes, including the senses, and learn how information is stored in biological systems. A maggot may be evolution's way of making a fly, but for scientists it provides us with an astonishing tool for understanding key aspects of how animals develop and behave.
Matthew Cobb FRSB is professor of zoology at The University of Manchester and author of Life’s Greatest Secret: The Race to Crack the Genetic Code.
Five centuries of maggot science
In the 1660s and 1670s, Dutch pioneer microscopist and natural historian Jan Swammerdam (1637–1680), tried to define what he called the “rules and theorems” of how insects grow. He noted the difference between insects that show a gradual transformation as they moult and grow, such as grasshoppers, and those such as flies and butterflies that have a larval and then a pupal stage before emerging as an adult.
Before the 1660s, it was generally assumed that the maggot died in the pupa, and that the adult fly was generated out of the decay of the maggot, much as maggots themselves were generated by the decay of meat or fruit. In the second half of the 1660s, experiments by Swammerdam and by Francesco Redi in Italy showed that maggots, in fact, came from eggs that were laid by a fly of the same species, and did not generate from rotting matter.
Swammerdam then used careful dissection to show that, in the case of the butterfly, key organs of the adult form were already contained within the caterpillar shortly before pupation. He generalised this principle to all insects, showing that the larval stage and the adult were the same organism.
Although Swammerdam was able to see more or less how a caterpillar turned into a butterfly, the fly maggot defeated him. He made some amazing dissections of the maggot nervous system, but he could find no trace of the adult form in the larva or in the pupa. This is not surprising, as the future adult tissues are just small unrecognisable blobs in the maggot – you need molecular techniques to be able to identify them.
US biologist Jim Truman, a modern Swammerdam, has spent most of his life trying to understand how maggots turn into flies – in particular, the links between the maggot’s nervous system and that of the adult fly. In a recent paper, he traced the development of the cells that control the movement of the fly’s legs – the fly has legs only on its three thoracic segments, whereas the maggot has motor control in each of its 11 body segments. The developing insect uses a mixture of positional information (each cell knows where it is) and temporal information (it knows how old it is) to decide what it is going to do next. This kind of fundamental biology will help us understand the patterns of development in all organisms.


