News
2023 national statistics on the use of animal in science released
- Details
- 11 September 2024
The Home Office has released the statistics for scientific procedures involving animals in Great Britain for 2023, with data showing that the total number of procedures was down 3% compared to 2022. Overall, animal research activity is at its lowest level since 2001. This could reflect a combination of factors, from a shift in research priorities and funding to an increasing focus on the use of non-animal alternatives.
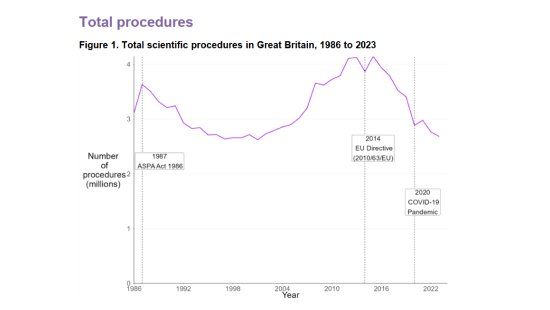
Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2023: data tables, Table 1.1 and Table 12.
There were a total of 2.68 million scientific procedures involving living animals completed in 2023 (this is usually higher than the number of animals used, as under certain conditions an animal may be reused).
1.47 million (55%) were experimental procedures, whilst 1.21 million procedures (45%) involved the generation or breeding of Genetically Altered (GA) animals.
92% of all procedures used mice, fish, birds or rats. The species distribution in experimental procedures is: 60% mice, 14% fish, 9.8% rats, 7.8% birds, 7% other species and 1.2% specially protected species. Procedures for generation of GA animals involved 86.3% mice, 13.1% fish, 0.44% rats and birds and 0.21% other species. The specially protected species are non-human primates, horses, dogs and cats, and applications to work with these species undergo additional scrutiny.
Animals were used in experimental procedures for the following purposes: around 770,000 procedures (52%) were for basic research, most commonly focusing on the nervous system, the immune system, and cancer. 21% of experimental procedures were for regulatory purposes, a similar figure to 2022. These procedures include safety and efficacy testing for new drugs and treatments before they are used in human clinical trials. 25% were for applied research, again a similar percentage to 2022. Applied research attempts to address diseases through prevention and development of treatments, most commonly to tackle animal diseases and disorders, human cancer, human infectious and neurological disorders.
All scientific procedures involving vertebrate animals (other than humans) and cephalopods must be performed under licence and the use of animals must be recorded, together with an assessment of the severity levels, and published every year in accordance with the Animals (Scientific Procedures) Act 1986 amended 2012.
Dr Mark Downs CSci FRSB, chief executive of the Royal Society of Biology, commented:
“The advancement of biological science and the development of biomedical treatments, for humans and animals alike, will require the regulated use of animals in science for the foreseeable future.
“Animal research remains a small but vital part of biomedical research dedicated to elucidating the mechanisms of infectious or non-communicable diseases, such as multiple sclerosis, stroke or dementia, or the testing of potential new treatments.
“The UK life science community aims to achieve the expected benefits of doing research with animals, while minimising the harms caused to them, by maintaining the highest standards of animal welfare and by applying the principles of the 3Rs: replacement, reduction and refinement of animal studies.
“Validated alternative methods, such as human cell-based systems, organ-on-a-chip and computational methods, are being phased-in to complement or replace animal use in an increasing number of applications.
“The UK has a strict regulatory system that requires scientists and laboratory staff to show competence before they can perform animal experiments. Each research project is individually assessed and licensed by the regulator, and reviewed by local ethical review bodies, before it can proceed.
“The Royal Society of Biology supports the use of animals in research when no alternatives are available, and is committed to promoting openness and transparency in reporting the use of animals for scientific purposes”.
Professor Clare Stanford FRSB, chair of the RSB Animal Science Group, commented:
“The publication of these data is a testament to the UK’s commitment to openness and transparency about the use of animals in scientific procedures.
“The total number of animals used in scientific procedures is the lowest on record: this reduction most likely reflects a reduction in charitable income and a shift in funding to the use of in vitro alternatives, which is now making important contributions to biomedical research.
“Research using animals to find cures for serious human illness, such as cancer, dementia and poor mental health will be needed for the foreseeable future. However, it is good to see a reduction in the severity of the harms experienced by the animals this year, which is further confirmation of researchers’ commitment to the 3Rs.”
For context, a procedure is defined as anything that causes pain, suffering, distress or lasting harm equivalent to, or greater than, the insertion of a hypodermic needle in accordance with good veterinary practice (for example a vaccination). This is the threshold. Even if the genetic modification causes no harm (i.e. is below threshold) breeding natural mutants or genetically engineered animals is counted as a procedure.
The actual severity of regulated procedures has been recorded since 2014. The actual severity of experimental procedures on animals were 97% sub-threshold, mild, moderate or non-recovery (where the animals does not wake up after anaesthesia), and only 3% were severe.
The actual severity of procedures for the generation and breeding of GA animals were 99% sub-threshold, mild, moderate or non-recovery, only 1% were severe. 1.11 million (91%) procedures used GA animals with no harmful phenotype.
Procedures are classed as “sub-threshold” when they do not cause suffering above the threshold for regulation. Mild severity is the equivalent of an injection or having a blood sample taken, moderate severity is greater than transient pain (for example surgery under anaesthesia followed by painkillers during recovery), severe suffering is something that we would not wish to endure (for example a heart attack).
Animal research in the UK is strictly regulated according to the policy and operations of the Animals in Science Regulation Unit.
The Society supports the efforts of the scientific community to replace, reduce and refine animal experiments (3Rs) and also advocates transparency in reporting the justification and outcomes of research involving animals. Members of the public can access a summary of each licensed research project when they are published on the Home Office website.
The Society discusses issues related to animal science through its special interest group the Animal Science Group, and is involved in informing policy for the advancement of science and animal welfare as a member of the UK Bioscience Sector Coalition.

